
Understanding the science behind hand sanitisers
With the outbreak of coronavirus, hand hygiene has gradually became a part of our daily life practice. In view of this, a free live webinar was co-organised by the Centre for Foundations Studies (CFS) and Division of Programme Promotion (DPP) from UTAR Kampar Campus on 17 June 2020, via Zoom and Facebook live.
Present at the webinar were Tham Yeong Shyue (the host) from DPP and CFS lecturers Lionel Keith Vytialingam (moderator), Ho Wai Yew (first panellist) and Chan Jer Jing (the second panellist). The objective of the webinar was to enlighten the public on the chemical composition of hand sanitisers and their mechanisms of action, as well as to display a virtual demonstration on how to formulate hand sanitisers at home.
The webinar was kick-started by Lionel where he briefly explained the title “The Science behind Hand sanitisers”, the background of the panellists, as well as the importance of using hand sanitisers against the coronavirus during the post Movement Control Order (MCO).
From left: Lionel, Ho and Chan
Ho began the talk by reminding the participants to differentiate the main ingredients of hand sanitisers as “-cidal” or “-static”. The concept “-cidal” on hand sanitisers refers to the ability to kill microorganism while “-static” refers to the concept that prevents the microorganism from multiplying (inhibit the growth of microorganisms).
There are two types of ingredients in the hand sanitiser, the active ingredients and inactive ingredients. The types of active ingredients in hand sanitiser are ethanol (Ethyl alcohol), isopropanol (Isopropyl alcohol) and propan-1-ol. The ethanol, isopropanol and propan-1-ol are bactericidal, fungicidal and viricidal which means they can kill microorganisms. However, they have no lingering effect to kill microorganism and cause drying of hands. The inactive ingredients, on the other hand, are Aqua (water), pH adjuster (aminomethyl propanol and triethanolamine, thickening agents (acrylates/C10-30 alky acrylate cross polymer and Xanthan gum), colouring, fragrance (parfum), denaturant (ethyl acetate, tertbutyl alcohol, denatonium benzoate, methanol), and other antimicrobial agents like chlorhexidine, benzalkonium chloride and triclosan.
“Some people might be addicted to alcohol. To prevent people from abusing alcoholic hand rub, governments throughout the world set a regulation to add in a kind of chemical denaturant, namely ‘methanol’ into the hand sanitiser to deter people from drinking it,” said Ho. He stressed on the dangers of “methanol” as it causes poisoning.
“Methanol could cause permanent blindness or death to the person who accidentally consumed it,” warned Ho and then he added, “If you are using the alcoholic hand sanitiser that contains methanol, please be aware to keep the hand sanitiser away from children and be more cautious when you apply it.”


Ho explaining the functions and types of ingredients in hand sanitiser
Ho continued his talk by explaining why it is important to use hand sanitisers that contain more than 60 per cent alcohol. According to the studies done by many researchers, alcohol-based hand sanitisers that contain 60-80 per cent alcohol are proven to be effective against many types of bacteria and viruses including Influenza, HIV and COVID-19. Bacteria and viruses can be killed in 10 seconds using hand sanitisers that contain 60-95 per cent alcohol. Ho said, “However, hand sanitisers with alcohol content up to 60 per cent only kill a certain type of microorganisms and not all viruses can be killed using alcohol. Viruses such as Hepatitis A and Poliovirus are very resistant to alcohol.”
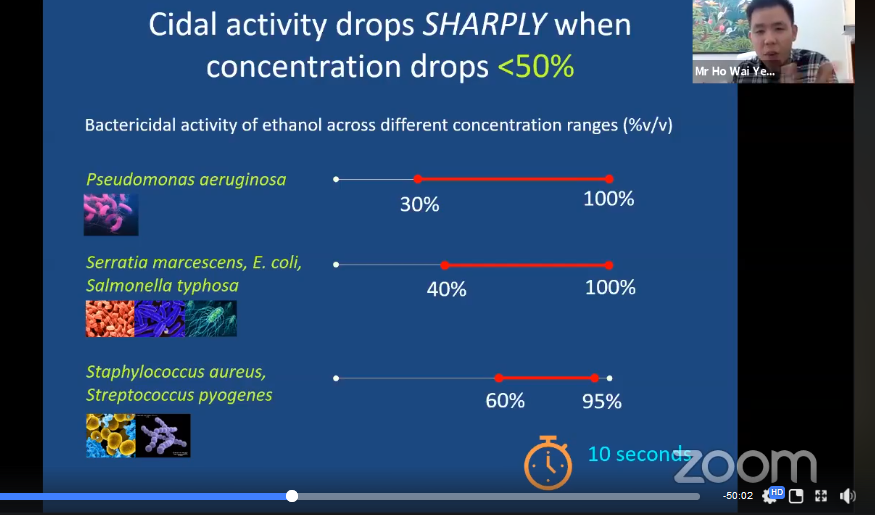
Ho displaying the bactericidal activity of ethanol across different concentration range (%v/v)
When asked about the effectiveness of using alcoholic beverages as hand sanitisers, Ho explained that alcoholic beverages like beer, red wine and vodka contain an insufficient volume of alcohol which could not kill viruses like COVID-19. Alcoholic beverage like beer contains only four to seven per cent alcohol, while most wine and liquor contain 12-15 per cent and 40-43 per cent alcohol respectively.
The talk was then continued by Chan, who demonstrated and explained how to make a hand sanitisers at home. Chan shared two formulas of hand sanitiser. The first formula contains 75 per cent alcohol which is largely recommended by the World Health Organization (WHO) whereas the second formula contains 60 per cent alcohol; it is recommended by the Australian Institute of Personal Care Science. The virtual demonstrations were shown through two videos which were pre-recorded earlier.
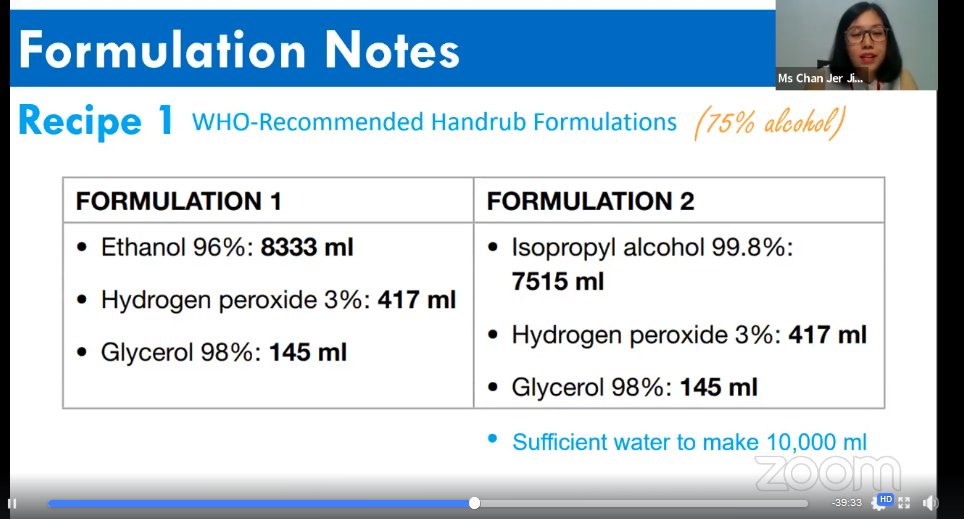
Chan showing the two types of formulations for hand sanitiser
According to Chan, WHO has recommended two types of formulations for hand sanitiser and the only difference between them are the type and volume of alcohol. Both formulations contain either 96 per cent of ethanol in 8,333ml or 99.8 per cent of isopropyl alcohol in 7,515ml combined with three per cent of hydrogen peroxide in 417ml and 98 per cent of glycerol in 145ml. Both formulations need sufficient water to make 10,000ml. For the demonstration, Chan chose the formulation with 99.8 per cent of isopropyl alcohol to make the hand sanitiser.
“For personal use, we only need 320ml of hand sanitiser in a bottle. To make the 320ml hand sanitiser, we need 1 cup of isopropyl alcohol (240ml), 1 tablespoon of hydrogen peroxide (15ml), 1 teaspoon of glycerol (five millilitres) and 1/4 cup of water (60ml),” explained by Chan.

Chan displaying the formulation to make a 320ml hand sanitiser
In the second video, Chan demonstrated the second formula of DIY sanitising hand gel which contains 60 per cent of alcohol. The ingredients were measured by weight.

Hand sanitiser formula recommended by the Australian Institute of Personal Care Science
Speaking of accuracy, the measurement using weight is more accurate than volume because volume is easily affected by temperature. Chan also stressed on the points where the content of alcohol must be above 60 per cent to kill the virus effectively. She suggested using hand sanitisers with 75 per cent of alcohol content as alcohol evaporates over time.
“When you purchase the alcohol, make sure they must be in food grade, cosmetic grade or medical grade. Avoid using essential oil apart from lavender and tea tree oil because other essential oil such as bergamot, bitter orange, lemon or grapefruit will increase the risk of sensitivity or allergy. Also, bear in mind that the water used to make the hand sanitiser must be boiled or autoclaved,” said Chan.
Chan also reminded the participants to make hand sanitisers for their own personal use because the hand sanitisers sold in the market require approval from KKM (Kementerian Kesihatan Malaysia).
“Using aloe vera gel with alcohol may not work in killing the virus,” said Chan before ending her session. The talk ended with a Q&A session.
Ho is currently pursuing his doctorate studies in UTAR Faculty of Science. His research focuses on the role of commensal bacteria in the human gut. He is also a UTAR lecturer in the Department of Science and Engineering under CFS. He is active in the field of microbiology and has published several ISI peers-reviewed journal articles.
Chan is currently a senior lecturer in the Department of Science and Engineering under CFS. She received her MSc in Plant Pathology from the School of Biological Sciences, Universiti Sains Malaysia (USM) in 2007. She is a dedicated academic staff who teaches pre-university Biology courses. She also actively involves in the development of curriculum and learning materials, integrates various forms of technology to improve students’ comprehension of different concepts, incorporates numerous hands-on activities and strategies to instil a love for biology in students.
The full video of the webinar was uploaded on the Facebook page “Utar4U”. Please click on the link https://www.facebook.com/watch/?v=908392412917703 if you are interested to watch the full video of the webinar. As of 22 June 2020, there have been 3.1k views on Facebook. Approximately 250 people have taken part in the webinar live on Facebook and Zoom.
![]()
![]()
![]()
![]()
© 2020 UNIVERSITI TUNKU ABDUL RAHMAN DU012(A).
Wholly owned by UTAR Education Foundation Co. No. 578227-M LEGAL STATEMENT TERM OF USAGE PRIVACY NOTICE