
A webinar titled “COVID-19 Laboratory Routine Tests and Biosafety Protection” was co-organised by College of Pathologists of Academy of Medicine Malaysia and Mindray on 14 May 2020. The webinar was hosted on Zoom.
Dr Ngeow introducing the
speakers
Introducing and kick-starting the webinar was Organising Chairperson Senior
Prof Dr Ngeow Yun Fong. She is also the Chairperson of
UTAR’s
Centre for Research on Communicable
Diseases. Speaking at the webinar were Prof Sergio Bernardini and Prof Ma
Lijuan.
Dr Sergio explaining the role of
laboratory tests
With the first
to speak, Prof Sergio spoke on “The Value of Laboratory Medicine during the
COVID-19 Outbreak”. He explained the role of laboratory tests as well as the
Human Alpha Coronavirus and Human Beta Coronavirus. There were two types of
Human Alpha Coronaviruses identified. The first type, HCoV-NL63, saw
patients displaying symptoms such as cold, Laryngotracheitis (CRUP) and
sometimes severe LRT infections among children and the elderly.
The HCoV-229E, on the other hand, saw
patients displaying cold symptoms and sometimes severe LRT infection among
children and the elderly.
Under the
Human Beta Coronavirus category, the HCoV-OC43 saw patients displaying cold
and severe LRT infections among children and the elderly, and the same was
found in HCoV-HKU1 patients. Other viruses identified included Severe Acute
Respiratory Syndrome 2004 (SARS-CoV), Middle East Respiratory Syndrome 2012
(MERS-CoV), and Severe Acute Respiratory Syndrome 2019 (SARS-CoV-2).
He said,
“Availability of protein structural information is an essential prerequisite
for the interpretation of biological phenomena. Now, only nsp5 protease is
available, although it is expected that many other structures will come
soon. In the meantime, homology modelling could provide preliminary
structural clues.”
He continued
by explaining the tools used for testing, which included microbiology tools,
serology and others. He also mentioned, “Samples should be obtained by using
a flocked swab, if available, to enhance the collection and release of
cellular material. Swabs that contain calcium alginate, wood, or cotton
should be avoided because they may contain substances that inhibit PCR
testing. Ideally, swabs should be transferred into a universal transport
medium immediately after sample collection to preserve the viral nucleic
acid. Early-morning posterior oropharyngeal saliva samples (coughed up by
clearing the throat) have also been assured as useful specimen types and
would not require the use of a swab.”
He went on to
speak on the types of methods used, namely Clusters of Regularly Interspaced
Short Palindromic Repeats (CRISPRs), Specific High Sensitivity Enzyme
Reporter Unlocking (SHERLOCK), DNA Endonuclease Targeted CRISPR Trans
Reporter (DETECTR), and Heating Unextracted Diagnostic Samples to Obliterate
Nuclease (HUDSON). Using medical samples from blood, urine and stool, the
release to protect target nucleic acids are done with HUDSON. RNA and DNA
amplification is done using recombinase polymerase amplification and/or
in vitro transcription, while
target nucleic acids detection and signal amplification are done using
SHERLOCK, SHERLOCK V2 and DETECTR.
He explained,
“Nabs elicit their protective activities in three main steps; firstly in
preventing the attachment of the virion to its receptors on targeted cells;
causing aggregation of virus particles; lastly inducing viruses lysis
through the constant (C) region of the antibody-mediated opsonisation or
complement activation.”
Towards the
end of the session, Prof Sergio advised for the utilisation of Artificial
Intelligence (AI) tools to handle pandemic in the future. He also saw the
benefits of telemedicine and how it should be considered further as a
strategy and operational plan guiding health care providers to switch to
outpatient teleconsultations and increase tele-expertise and remote patient
monitoring. He also noted that telemedicine could facilitate data-sharing
mechanism to integrate telemedicine providers’ data with epidemiological
surveillance. He also shared ways on overcoming the lockdown and advised
everyone to stay safe.

Prof Ma explaining he common test conducted in laboratory testing
The next to
speak was Prof Ma. She spoke on “Blood Routine Test and Laboratory Safety
Precautions”. Her talk was divided into two parts; the first part saw common
test conducted in laboratory testing including blood routine test and
C-reaction Protein Test (CRP) while the second part saw laboratory
bio-safety protection during epidemic setting in term of personnel and
laboratory prevention.
Prof Ma
explained the application of Blood Routine Test & CRP for infected diseases
and COVID-19 diseases. “We need to use the correct application for blood
routine test. According to the epidemic review by the World Health
Organization (WHO), these infected diseases are ranked Top 4 around the
globe and it will cause death. Lower respiratory tract infection is the
fourth leading cause of death in the world and nearly 13 million children
die every year due to infectious disease, making it number one cause of
death among children in developed and developing countries,” said Prof Ma.
She continued
sharing the clinical application of CRP test where it is used for bacterial
infection, virus infection and mycoplasma infection. Prof Ma then explained
the treatment plan recommendation for COVID-19. She briefly explained the
Chinese Guideline of Diagnosis and Treatment of COVID-19 that involved the
process of laboratory examination, therapeutic monitoring and early clinical
warning.
Prof Ma also
emphasised, “COVID-19 also causes severe illness to children with 73
reported cases in New York with symptoms similar to Kawasaki disease and
toxic shock–like syndrome. Children would suddenly suffer from a rare and
fatal disease with symptoms like having rashes, carditis, and artery
swelling. Some even experienced death. And they were all tested positive for
COVID-19 test.”
She later
explained how COVID-19 is transmitted. The transmission includes direct
transmission, aerosol transmission and contact transmission. Aerosol
transmission poses the highest risk for laboratory personnel due to the mix
of droplets in the air. This could cause infection once inhaled. She added,
“Laboratory personnel usually come in contact with blood, urine, faeces and
other samples which could potentially produce aerosols. In the process of
blood collection, syringe, centrifugation, uncapping, sample adding,
automatic instrument and equipment operation, aerosol can be generated.”
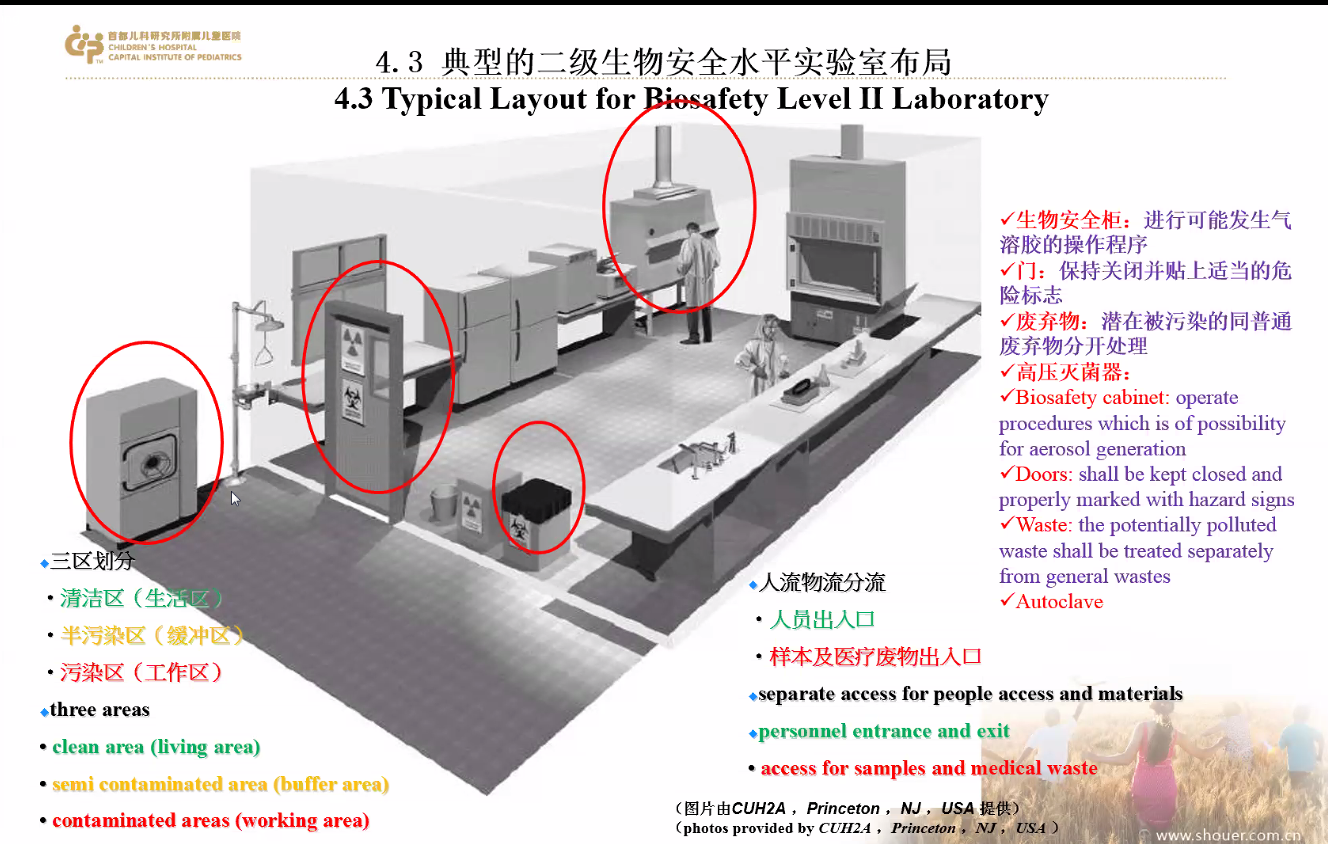


Prof Ma showing participants the
layout of biosafety laboratory during the epidemic
Towards the
end of the session, Prof Ma shared the importance of having laboratory
biosafety equipment and facilities to prevent aerosol splashing and reduce
diffusion. Laboratory biosafety equipment and facilities included biosafety
cabinet, autoclave, ultraviolet lamp, emergency shower equipment and
personal protective equipment such as face mask, medical cap, goggles, shoes
cover and isolation gown.

Staff technical training, biosafety training and assessment for the safety of laboratory personnel
The
webinar was adjourned by a Q&A session between the speakers and the
participants.
![]()
![]()
Wholly owned by UTAR Education Foundation small>Co. No. 578227-M LEGAL STATEMENT TERM OF USAGE PRIVACY NOTICE