
Chemical engineering professor shares research finding on MOF based electrocatalysts for electrolytic water splitting
Prof Lu delivering an informative lecture on MOF, electrocatalysts and electrolytic water splitting
UTAR Institute of Postgraduate Studies and Research (IPSR) organised a webinar titled “Metal Organic Framework (MOF) Based Electrocatalysts for Electrolytic Water Splitting” on 5 November 2020 via Zoom. It was a part of the UTAR International Collaboration Partner (ICP) webinar series.
The webinar was conducted by National Tsing Hua University Department of Chemical Engineering Tsing Hua Chair Professor Lu Shih-Yuan. He was also one of the International Collaboration Partners of UTAR Global Research Network. The moderator for the session was Division of Community and International Networking Director Assoc Prof Dr Lai Soon Onn.
Dr Lai (right) thanking Prof Lu for conducting the lecture
Prof Lu completed his PhD studies at the University of Wisconsin, USA and his master’s and degree in chemical engineering at National Taiwan University. His research interest included the preparation and characterisation of nanomaterials and nanostructures and their application in energy, photocatalyst and sensing. He has also published numerous journal articles and books in the field of his expertise over the years.
Firstly, Prof Lu briefed the outline of his research which included “Electrolytic water splitting to produce hydrogen” and “The need to develop cost-effective highly efficient and stable electrocatalysts”. He said, “The world is now facing two grand challenges which are the depletion of fossil fuels and over emission of carbon dioxide (CO2). The over emission of CO2 mainly occurred from the overconsumption of fossil fuels which led to the increase of CO2 concentration in the atmosphere; thus producing global warming and extreme climate. Therefore, clean alternative energies are needed to achieve a low carbon economy.”
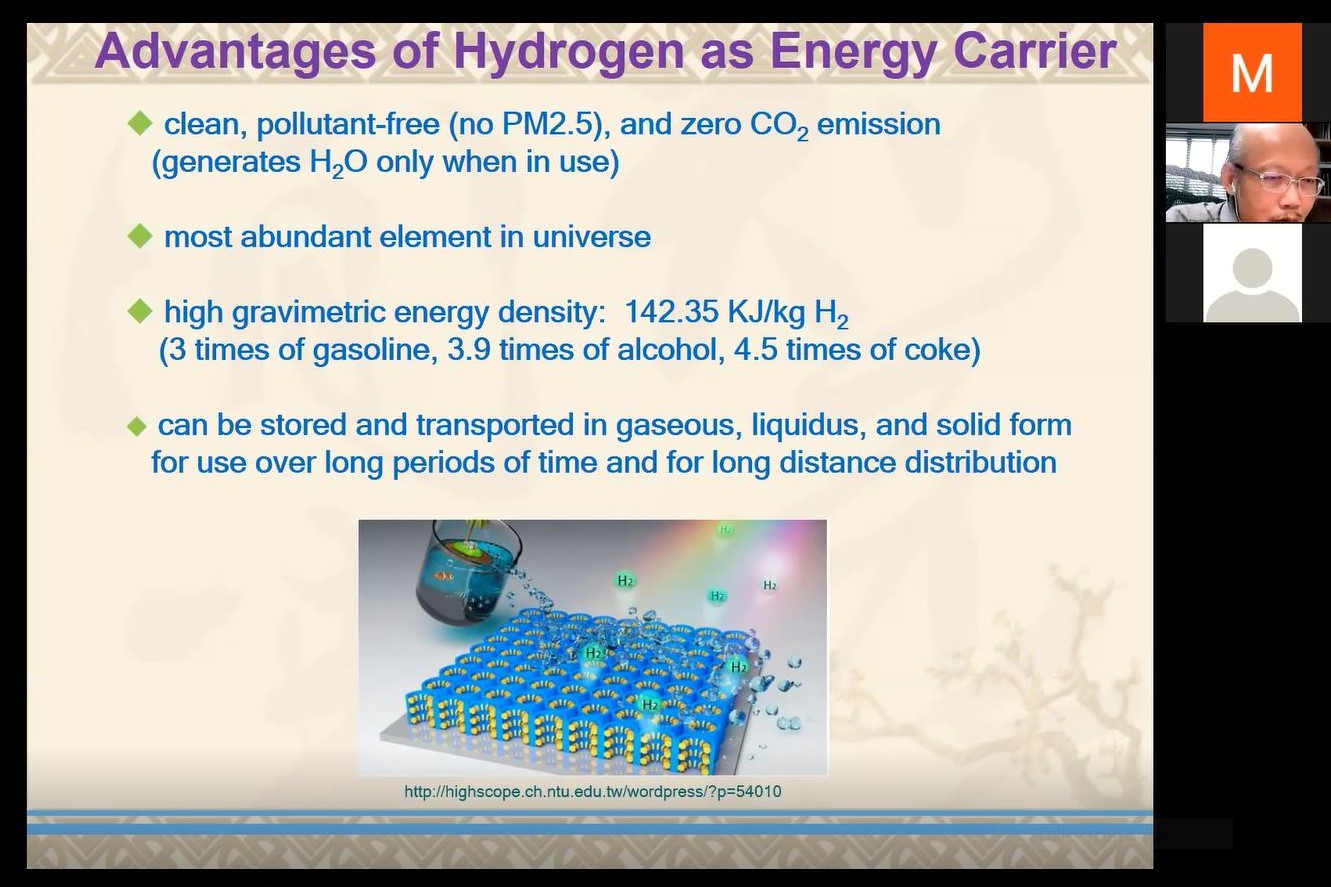
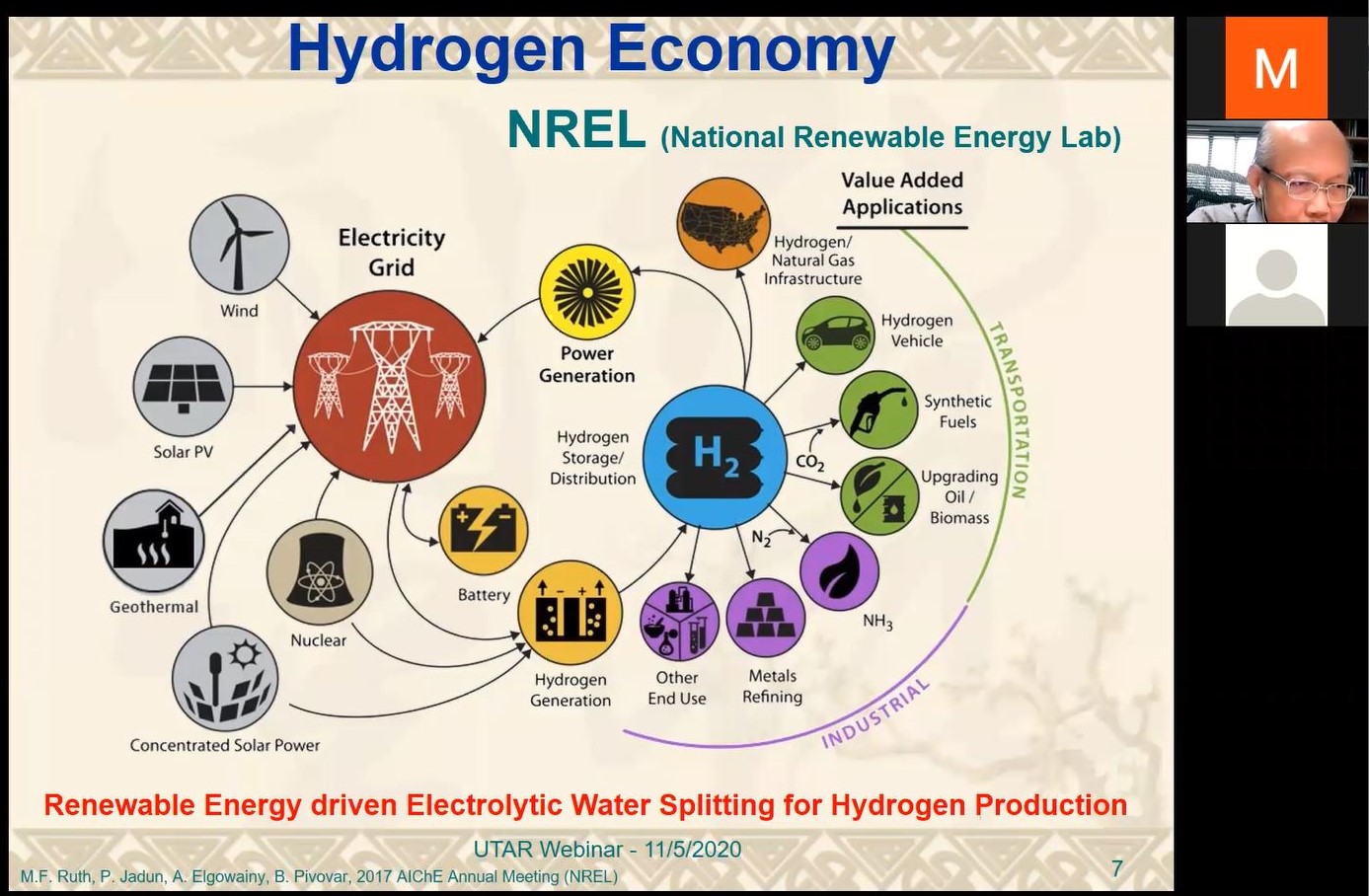
Prof Lu explaining the advantages of hydrogen as the energy carrier and hydrogen economy
Speaking of the characteristics of hydrogen, he explained, “Hydrogen is a good energy carrier because it is clean, pollutant-free (no PM2.5) and has no CO2 emission. It also comes with high gravimetric density (142.35KJ/kg H2 - 3 times of gasoline, 3.9 times of alcohol and 4.5 times of coke) and is known as the most abundant element in the universe. Most importantly, it can be stored and transported in gaseous, liquids, and solid form for long-period use and long-distance distribution.” He also shared how renewable energy drove the electrolytic water splitting for the hydrogen economy; the principle of electrolytic water splitting; ideals of electrocatalysts which are highly efficient, highly stable and incur low material cost; and the key strategy for catalyst development.
Elaboration on the principle of electrolytic water splitting, ideal electrocatalysts and key strategy for catalyst development
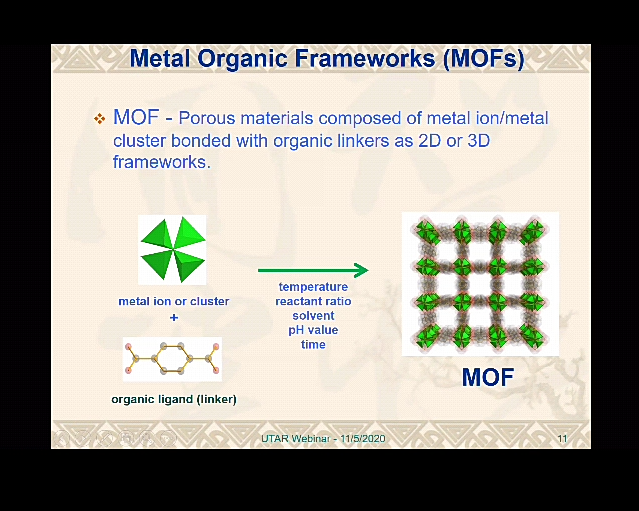
Introduction to the nanostructure of MOFs
Later, he introduced the metal organic frameworks (MOFs) and its application, saying, “MOF is a kind of porous materials composed of metal ion or metal cluster bonded with organic linkers such as 2D or 3D frameworks. With characteristics such as large surface and volume ratio, high surface area, stability, design flexibility, electrochemical character and high porosity, it can be used in multiple fields including catalysis, energy production and many more. Most MOFs are not water stable and are poor electrical conductors.”
He then further elaborated the MOFs for energy applications; the cons of MOF derived electrocatalysts; in-situ grown MOFs on porous conductive substrates; and oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) electrocatalysts of MOFs, oxides, alloys and sulfides, phosphides. Meanwhile, he analysed a few of the MOFs’ research examples to make it easier for participants to understand.
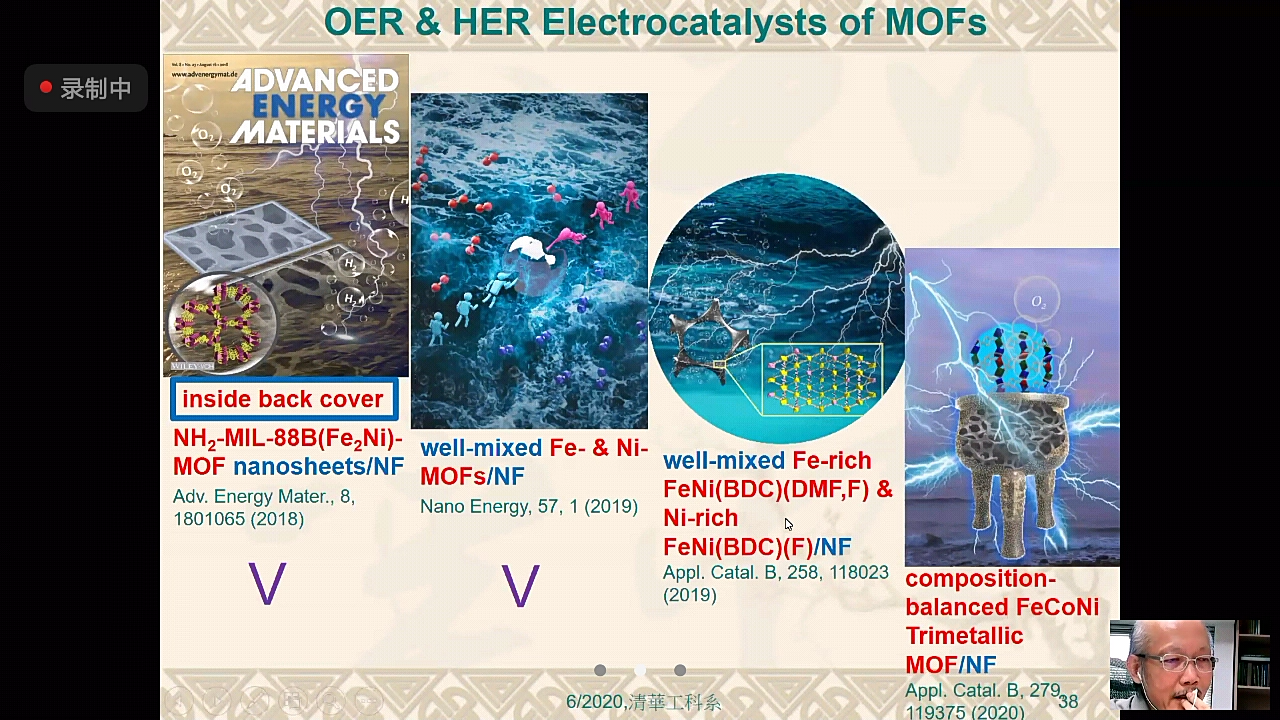
Prof Lu explaining the OER & HER electrocatalysts of MOFs
“The demonstration showed that the MOF/nickel foam (NF) composites as bifunctional electrocatalysts for water splitting, and not only materials are important, nanostructure and synergistic effects also play an important role. The results also showed that nanosheets are bigger than micron-sized spindle crystals, while well-mixed MOFs are better than usual mixed MOFs,” he concluded.
The comparison between nanosheets and micron-sized spindle crystals
The comparison between well-mixed MOFs and mixed MOFs
During the Q&A session, Prof Lu discussed topics such as the limitations and challenges for the development of nanostructure MOF for water splitting, the range of reading to prove whether the particular catalyst is effective for water splitting, criteria of water for water splitting and many more.
The UTAR Global Research Network is a network that links academics, researchers, scientists and technologists at the forefront of research and technology development who aspire to be or already involved or associated with the education and research activities of UTAR.
![]()
![]()
![]()
© 2020 UNIVERSITI TUNKU ABDUL RAHMAN DU012(A).
Wholly owned by UTAR Education Foundation Co. No. 578227-M LEGAL STATEMENT TERM OF USAGE PRIVACY NOTICE