

Panel speakers
With the aim to share knowledge on
the different types of stem cells and their potential applications in
research and therapy, UTAR
Centre for Stem Cell Research (CSCR) and Majlis
Kanser Nasional (MAKNA) jointly organised a webinar titled “Stem Cells: From
Bench to Clinic" on 8 January 2021.
The webinar which was hosted at Microsoft Teams saw more than 100
participants comprising of post-graduate students and researchers who are
working in the stem cell field.
CSCR Chairperson Prof Dr Alan Ong
Han Kiat welcomed the participants and speakers, “I’m delighted to have with
us our esteemed speakers in sharing knowledge and expertise to our
participants. At the same time, I would also like to thank all of you for
joining us today and wish all of you a fruitful session from this webinar.”
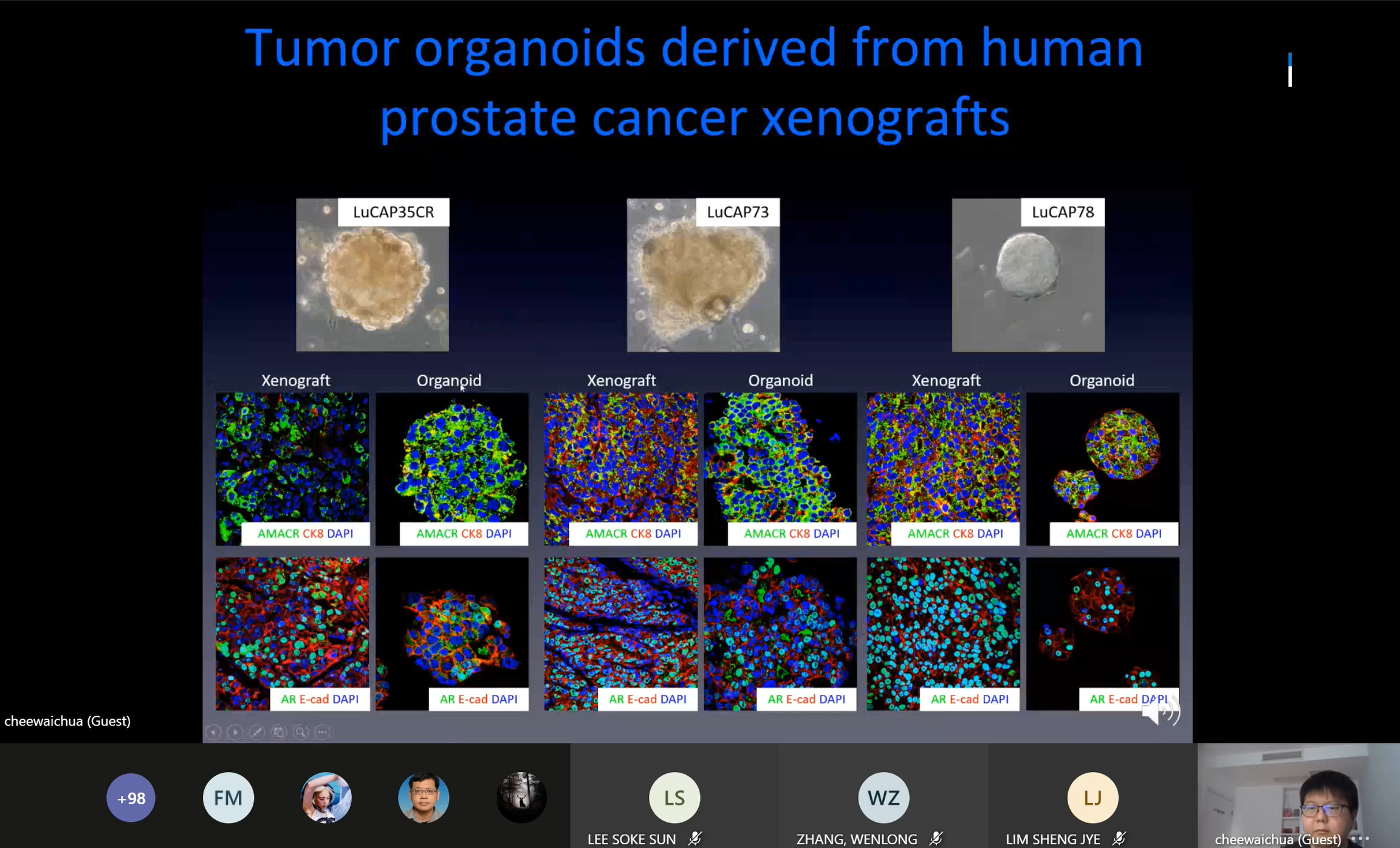
Prof Chua
explaining the tumour organoids derived from human prostate cancer
Shanghai Jiao Tong University,
China Prof Chua Chee Wai presented on the “Application of the organoid
technology in the study of prostate stem cell and cancer biology” during the
webinar. He said, “One of the major obstacles in the study of prostate
luminal progenitors and cancer cells is the lacking of in vitro assay
for luminal progenitors and cancer cells because these cells are difficult
to culture. Therefore, prostate cancer research is restricted by a handful
of established cell lines, thus hampering the study of tumour heterogeneity,
drug responses and stromal-epithelial interactions. The ideal
in vitro culture system should
carry three characteristics, which are long term maintenance and propagation
of luminal population, the ability to preserve androgen responsiveness and
AR signalling and the capability to integrate different stromal components.”
He spoke about luminal features of the prostate, novel organoid culture
assay, serial regression and regeneration and other related topics.
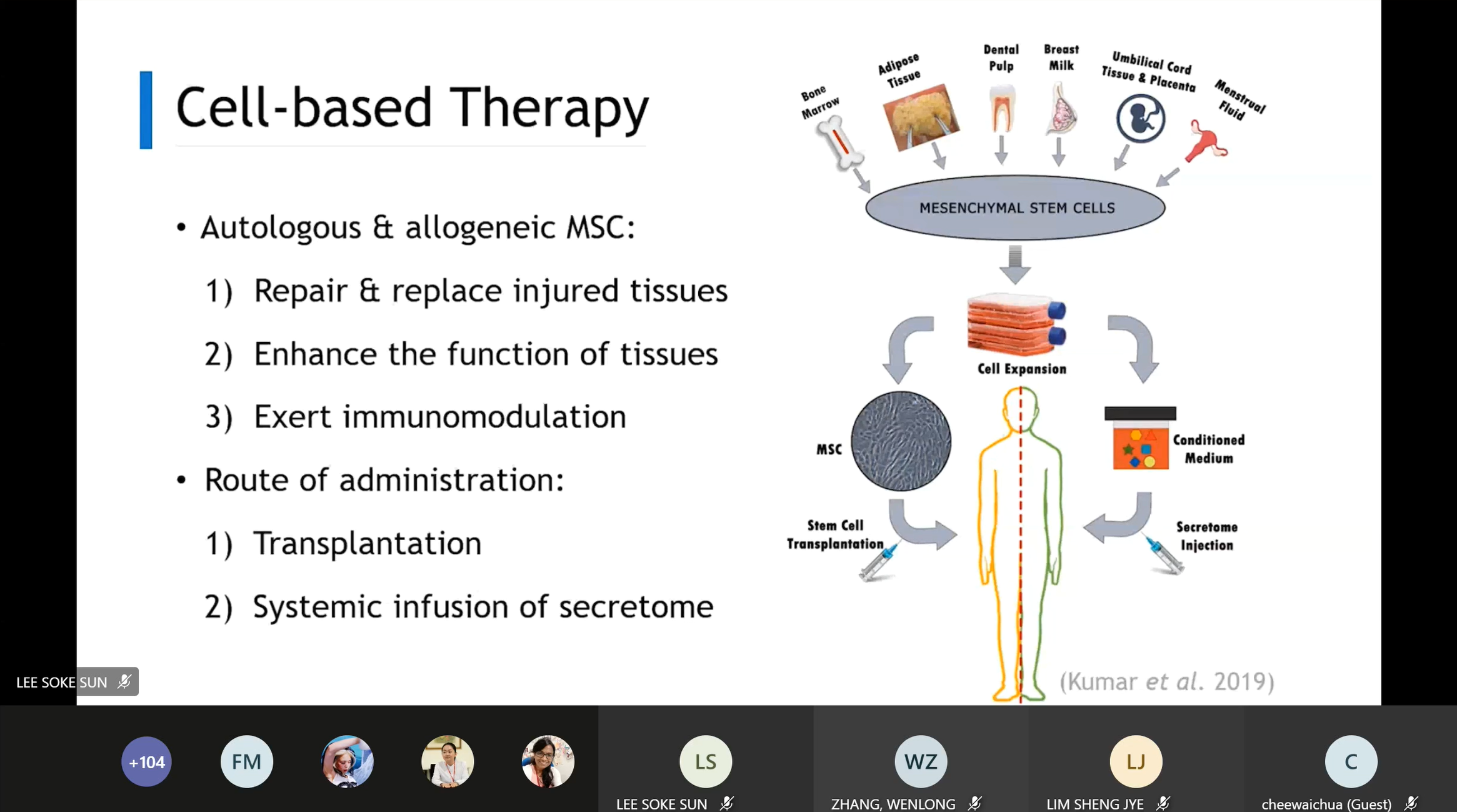
Dr Teoh sharing her
knowledge on cell-based therapy
The second sharing session was
presented by FMHS Dr Teoh Hoon Koon who spoke on “Mesenchymal stem cells:
Application in cell and gene therapy”. She shared about mesenchymal stem
cells, therapeutic properties, mechanism of action, MSC-based therapy,
genetically modified MSC and the challenges of MSC-based therapy. “MSC have
distinct characteristics that make them an attractive candidate in
cell-based therapy for the treatment of human diseases. They are easy to
isolate and cultured in the lab and produced in a large scale for clinical
usage. MSC also has the homing ability to the site of tissue or organ damage
while exhibiting little or low immunogenicity. Moreover, MSC secretes
bioactive substances that regulate the local cellular responses to injury.
Some of the challenges of MSC-based therapy in the clinical application are
the heterogeneity of MSC, side effect from administration of exogenous MSC,
short survival of administered MSC and pro-tumour activity,” said Dr Teoh.
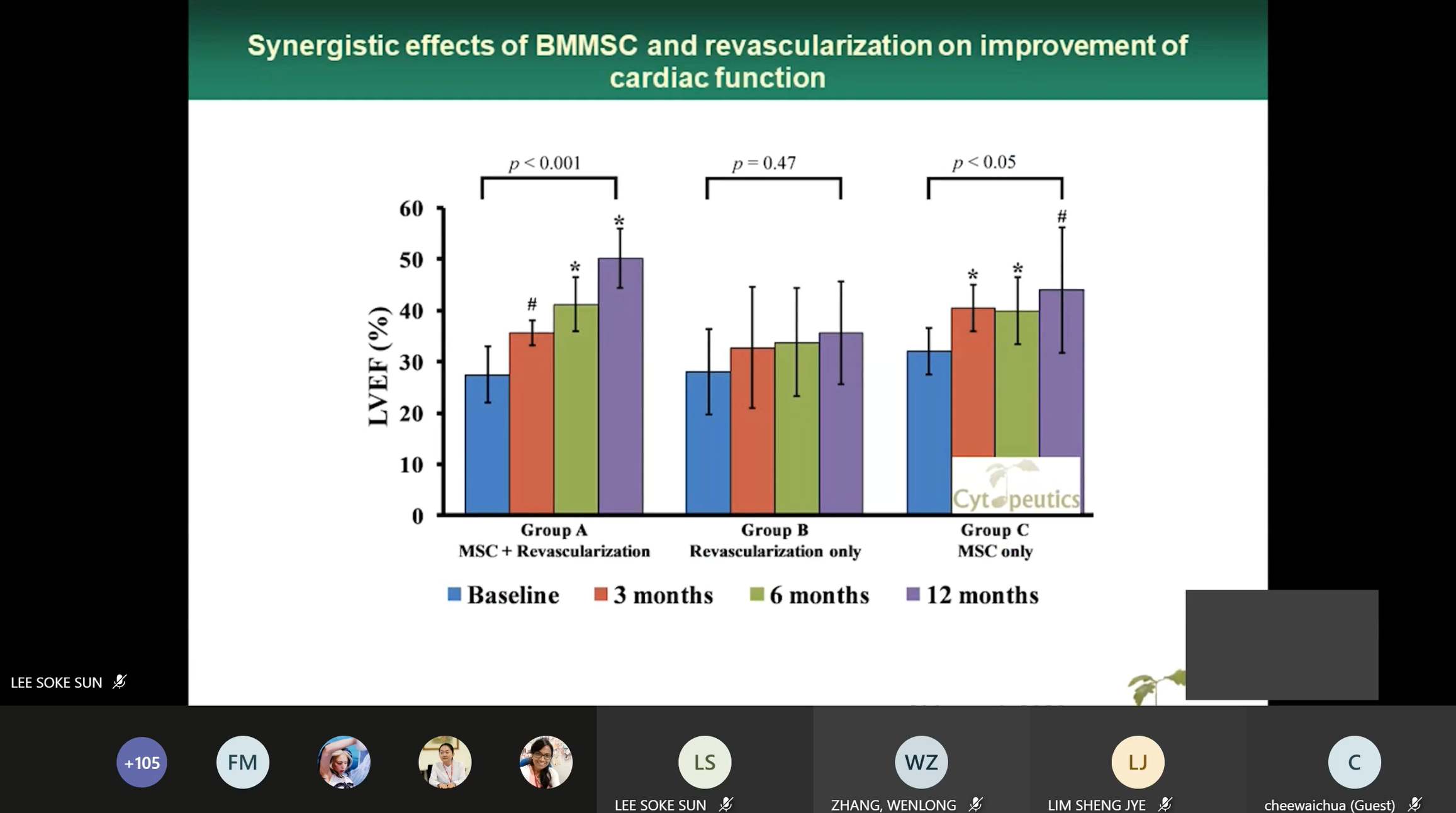
Dr Wong speaking on
the synergistic effects of BMMSC and revascularisation
The webinar was followed by a
presentation by Cytopeutics Sdn Bhd Malaysia Dr Wong Chee Yin on “Current
development of stem cell therapy in Malaysia: A perspective from a private
company”. He gave an introduction on Cytopeutics Sdn Bhd which conducted
research on Mesenchymal stem cells for more than 10 years and spoke on MSC
treatment for heart disease, clinical trial on autologous BM-MSCs treatment
for chronic severe dilated cardiomyopathy, MSC treatment for critical limb
ischemia, acute stroke and intervertebral disc disease as well as the
difference between autologous and allogenic. He said, “The current treatment
procedure for heart disease are angioplasty and bypass, however, there are
limitations to these two procedures. These procedures can only supply the
blood back to ischemic regions but do not regenerate heart tissues.
Autologous need time to culture, and some patients may not be suitable for
BMA or tissue extraction for MSC isolation and the elderly person has lower
potency MSC while allogenic can be found off the shelf and, can find a fit
and better donor.”
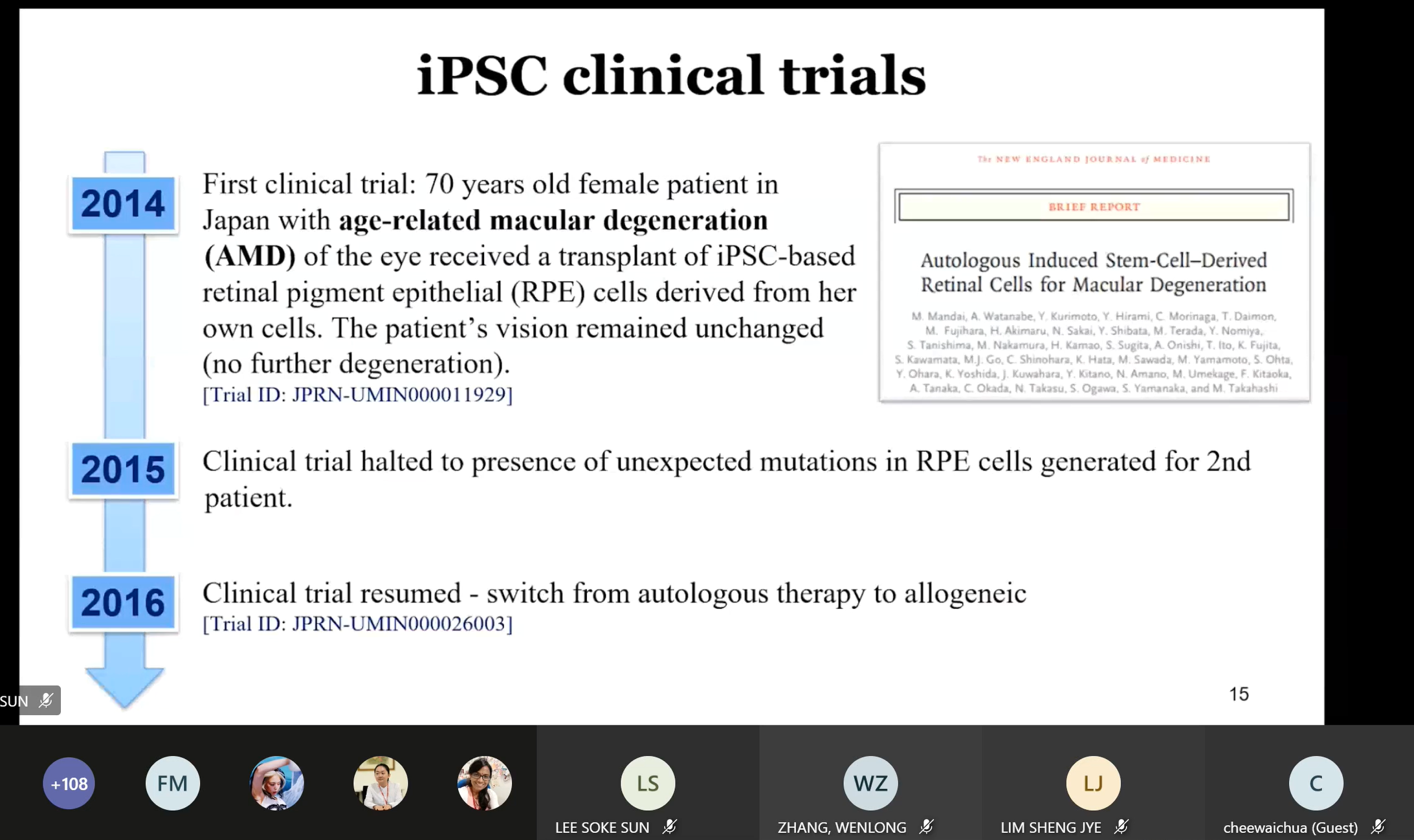
Tai presenting the
iPSC clinical trials since 2014
Cytopeutics Sdn Bhd Malaysia Tai
Li Hui shared on “iPSC technology: Their role in regenerative medicine”
where she introduced induced pluripotent stem cells (iPSC), differentiation
of potential of iPSC and iPSC clinical trials. “The two key characteristics
of iPSC are self-renewal capacity and it is differentiated into specialised
cell types of endoderm, mesoderm and ectoderm. iPSC has shown great
application in four major fields known as regenerative medicine and
cell-based therapy, disease modelling, drug discovery and human
developmental biology. The challenges in iPSC application are such as
derivation of clinical-grade iPSC and iPSC-derived therapeutic cell
products, genomic instability, the risk of potential tumorigenicity, immune
rejection complication, phenotypic heterogeneity of the therapeutic cell
products and large cohort of iPSC line for polygenic and sporadic disease
aetiology,” said Tai.
Dr Erica explaining
the reprogramming cells
Majlis Kanser Nasional Malaysia
Senior Scientific Officer Dr Erica Choon Pei Feng presented on
“Cancer-derived iPSC as cancer model: An overview” during the last sharing.
She spoke about iPSC, cancer cells reprogramming, pancreatic ductal
adenocarcinoma, glioblastoma-derived iPSC, non-small cell lung cancer,
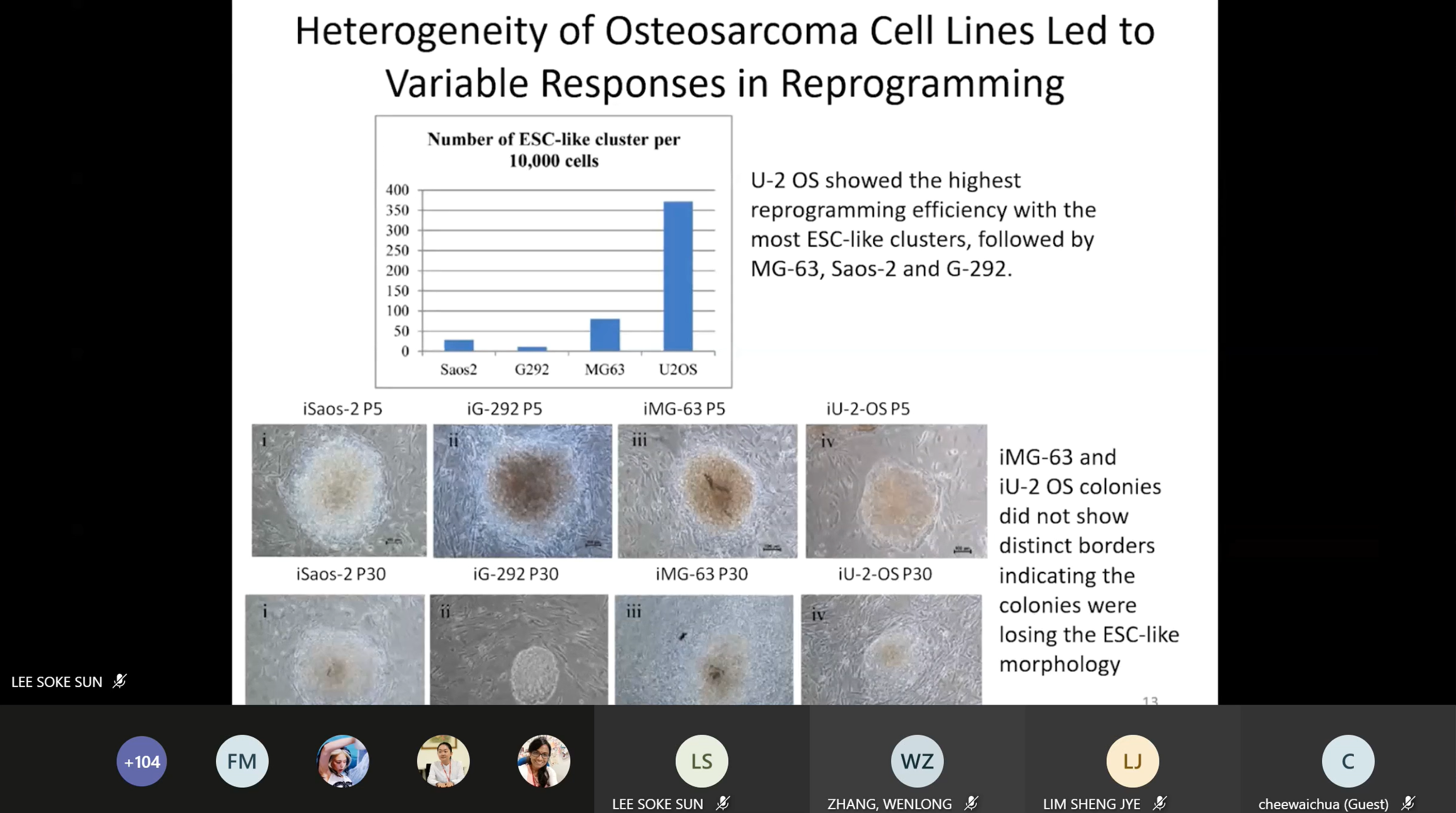
chronic myeloid leukaemia, osteosarcoma and other related topics. She said,
“The advancement of reprogramming of cancer cells opened an enormous
opportunity to study tumour development and progression as well as drug
therapy development. Gene expression and functional data suggested that the
involvement of GAADD45G in OS development and progression that remains to be
elucidated. Further study involving overexpression of GADD45G in OS and
other cancer cells may provide more important information on the role of
GADD45G in OS, as well as to study the progression of OS from OS-iPSC to
differentiation into terminal osteoblast and to elucidate the aberrations in
oncogenes expression during OS development.”
![]()
Wholly owned by UTAR Education Foundation Co. No. 578227-M LEGAL STATEMENT TERM OF USAGE PRIVACY NOTICE